This Plant Cutting is devoted to the chemical side of things, both the synthetic capabilities of the Plant Kingdom, and the ingenuity of humans who recognise exploitation potential in one of the most fundamental of plant compounds. So, we have items on a pesticide from ferns, and a new class of human-engineered, plant-derived polymers. Read on…
Phern-produced pesticide

This image, captioned “Pteris cretica ‘Albolineata’. Vegetative leaves and sporophylls (upper, linear)”, is by Ping an Chang and is used under the Creative Commons Attribution-Share Alike 4.0 International license.
When we think of useful chemicals from plants – it we ever do, that is – it’s most likely flowering plants that come to mind. True, they contain a myriad of compounds, and some of those have been harnessed by humankind for useful purposes (Manuel Balandrin et al., Science 228(4704): 1154-1160, 1985; doi: 10.1126/science.3890182; Melissa Petruzzello; Natalia Carreno-Quintero & Pablo Cárdenas (2021) Front. Young Minds. 9:512423; doi: 10.3389/frym.2020.512423).
But, angiosperms are not the only plants that possess potentially people-useful chemicals. Take for example, ferns, and in particular Pteris cretica cv. albolineata, sometimes called Cretan brake fern, or silver ribbon fern. Although it is apparently a common houseplant (Elizabeth Pennisi), it’s an otherwise reasonably unremarkable plant. However, work by Jun-Zhi Wei et al. (PNAS 120(44): e2306177120; https://doi.org/10.1073/pnas.2306177120) shows that pteridophyte in quite a different light*.
Wei et al. have extracted from the fern what they describe as a new family of insecticidal proteins. In other words, they have identified a new group of chemicals/compounds that kill insects. And, since, insects are major pests of crop plants (Katie Avis-Riordan), anything that will help people in humanity’s age-old battle to protect food plants from the unwanted attentions of these invertebrates has to be good news.
Whilst the discovery of novel compounds from nature is always a matter of some interest, most noteworthy in this instance is the fact that the molecules resemble the insecticidal proteins produced by the bacterium Bacillus thuringiensis (Mohamed Ibrahim et al., Bioengineered Bugs 1(1): 31–50, 2010; https://doi.org/10.4161/bbug.1.1.10519; Gholamreza Jouzani et al., Appl Microbiol Biotechnol 101: 2691–2711, 2017; https://doi.org/10.1007/s00253-017-8175-y; Eric Vinje). The genes for these so-called Bt [from the initial letters of the two-part scientific name of the microbe] toxins have been genetically engineered (Mike Smith) into a range of crops to give them in-built resistance to a range of insect pests [and provides the ‘Bt’ in the names of Bt cotton, and Bt maize/Bt corn].
Are the new so-named IPD113 proteins similarly effective as insecticides? When fern extracts were added to artificial diets, growth of soybean looper (SBL, Chrysodeixis includens) (Ethan Carter & Jennifer Gillett-Kaufman), and corn earworm (CEW, Helicoverpa zea) (John Capinera) was stunted, but there was little or no effect against European corn borer (ECB, Ostrinia nubilalis) (John Capinera), fall armyworm (FAW, Spodoptera frugiperda) (John Capinera), and western corn rootworm (WCR, Diabrotica virgifera virgifera). Leaves of maize genetically-modified with IPD113 protein were strongly protected against CEW and ECB, and moderately protected against FAW; ears and stalks were also protected against CEW and ECB. Leaves of transgenic soy displayed good protection against CEW, SBL, VBC, and FAW.
Taken together, these results are encouraging and indicate that the IPD113 compounds have considerable potential/promise as plant-pest-protecting proteins. Which is most encouraging because, although Bt crops have had considerable success at warding-off the unwelcome attentions of insect pests (Matthew Niederhuber; Wilhelm Klümper & Matin Qaim (2014) PLoS ONE 9(11): e111629; doi:10.1371/journal.pone.0111629; Dan Charles), there are concerns that insects are becoming resistant to the Bt toxin (John Roche; Richard Hellmich & Kristina Allyse Hellmich; Bruce Tabashnik & Yves Carrière). Despite some chemical similarity to the Bt protein, IPD113 appears to act in a dissimilar way. Discovery – and development – of an alternative to Bt toxin that should overcome insect-resistance to that bacterial-derived insecticide is to be welcomed.
[For more scicomm insights into this work, see here, here, here, Arpita Yadav, and the article by the ever-reliable Elizabeth Pennisi.]
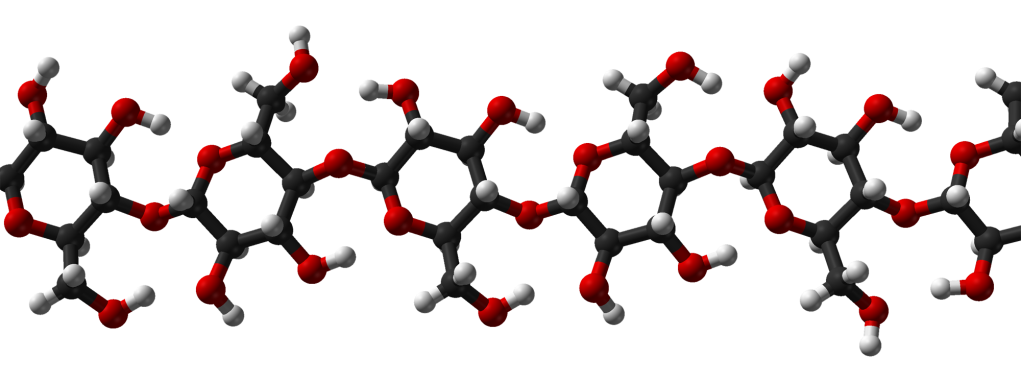
This image, entitled “Ball-and-stick model of part of the crystal structure of cellulose Iβ”, by Ben Mills, is in the public domain.
One of the most abundant organic materials on the planet is cellulose. Although this chemical is the main strengthening component of plant cell walls (Bruce Alberts et al., Molecular Biology of the Cell. 4th edition. New York: Garland Science; 2002), it is essentially a very long molecule that’s constructed from thousands of glucose molecules (Charles Ophardt) chemically-linked together. The individual units of glucose in such a chemical are termed monomers (Johnnie French), and the entity they give rise to – the cellulose molecule – is a polymer (Alina Bradford)**.
In nature, cellulose is broken down when plant material is decomposed and is its component atoms recycled back into the bodies of living organisms. Humans often disrupt that cycle by burning appropriate plant material as biomass fuel (G Philip Robertson et al.) to provide an alternative energy source to combustion of fossil fuels. That use may now have competition from the work of Yuta Mizukami et al. (ACS Macro Lett. 13(2): 252–259, 2024; https://doi.org/10.1021/acsmacrolett.3c00720)*** who have discovered how to make stable polymers from cellulose that offer a sustainable alternative to conventional plastics.
However, rather than use cellulose directly, Mizukami et al. used levoglucosenone (LGO)**** and dihydrolevoglucosenone (Cyrene), small molecules which are commercially-available and derived from cellulose. Just as non-sugar plastics have a myriad of uses, the newly-created polymers are anticipated to have use in a variety of ways, as alterations to the precise chemical structure of the polymers will generate different materials for a range of possible applications. Of particular interest is the fact that the synthesised polysaccharides have high thermal stability and form amorphous solids under ambient conditions, which have been processed into highly transparent self-standing films.
Although Mizukami et al. (2024) have produced what they call ‘unnatural’ polysaccharides, that adjective (Eoghan Ryan) has more to do with the fact that these molecules aren’t found in nature – i.e. aren’t ‘natural’ – rather than having undesirable qualities (as far as is known). Indeed, and as unnatural as they may be, these new polymers have a major advantage over traditional plastics in that they are recyclable [much like the cellulose from which they are ultimately derived], which quality is highly desirable. Given the current – and future – problems that arise from humanity’s obsession with plastics that are not always recyclable or recycled (Deepa Fernandes & Adeline Sire), and that cause considerable concerns for the health of humans (Eryn Gold; Zana Shabani Isenaj), other living things and the environment when these materials are present in nature, this work is something to take notice of.
Hopefully, Mizukami et al’’s new bioplastics (Fabio Lamberti et al., J Polym Environ 28: 2551–2571, 2020; https://doi.org/10.1007/s10924-020-01795-8; Jan-George Rosenboom et al., Nat Rev Mater 7: 117–137, 2022; https://doi.org/10.1038/s41578-021-00407-8) will soon take their place amongst the increasing number of sustainable polymers from renewable sources (Yunqing Zhu et al., Nature 540: 354–362, 2016; https://doi.org/10.1038/nature21001; Amar Mohanty et al., Nat Rev Methods Primers 2, 46 (2022); https://doi.org/10.1038/s43586-022-00124-8).
[For more scicomm items about this work, see here, here, here, and here.]
* Another noteworthy property of this fern is its ability to hyperaccumulate the harmful metalloid arsenic (Marek Popov et al., Ecotoxicology and Environmental Safety 216, 15 June 2021, 112196; https://doi.org/10.1016/j.ecoenv.2021.112196)…
** And, because glucose is type of organic compound (Brad Basehore et al.; Melvin Usselman et al. known as a saccharide (or simple sugar), the completed cellulose molecule – consisting of many, many such single, monosaccharide units of glucose – is a polysaccharide (Charles Ophardt). [For completeness, it is worth adding that glucose is also a carbohydrate (James Benton (which term is used as an alternative to saccharide in biochemistry), a simple one – and cellulose is therefore a complex carbohydrate (Suzanne Wakim & Mandeep Grewal)…]
*** Even though the advanced synthetic organic chemistry terminology may be beyond my understanding and needs, you’ve got to like a research paper that contains such phrasing as “To our delight, when the reaction was quenched with Ac2O in pyridine with a catalytic amount of 4-dimethylaminopyridine (DMAP), P1 with Mn,SEC = 7.0 kDa and Đ = 1.46 was obtained (run 4 in Table 1)”. Can’t you just feel the authors’ excitement at their discovery? Lovely stuff.
**** Is it just Mr P Cuttings who is struck – and more than a little amused – by the similarity between using repeating units of LGO to make larger plastic-like structures and constructing entities from plastic ‘monomers’ of LEGO..?

Leave a comment